The effect of detergents on plants
Year 9-10 Practical Report Example
Aim
To investigate the effect of detergent on the health of plants when they are introduced in increasing amounts to the water supply.
Introduction
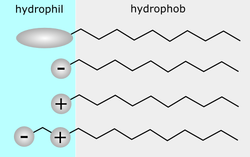 Figure 1: Surfactants (Roland.chem, 2006) Figure 1: Surfactants (Roland.chem, 2006)
The experimental investigation will examine the effects of detergents on the health of an Elodea plant. Detergents are sometimes known as soaps, and are made of molecules that have a hydrophilic head that is attracted to water, and a hydrophobic tail that is attracted to oils and grease (see figure 1) (Taylor, 2007, p.226). This makes detergent so effective when cleaning grease off of dishes because the tail can attach to the oils and grease on the plate while the head can attach to the running water to pull the grease away.
|
Key concepts to cover in the introduction:
|
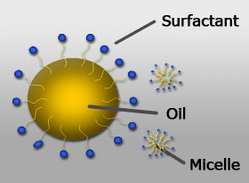 Figure 2: Oil micelles (Northeastern University, 2009)
Figure 2: Oil micelles (Northeastern University, 2009)
When there is enough detergent molecules separate the oils and grease, they can surround them as per figure 2, such that only the hydrophilic heads are exposed on the outside. This formation is called a Micelle (Taylor, 2007, p.234). This keeps the oils dissolved in water, and prevents the oils from clumping together again since the charges on the hydrophilic heads repel each other.
Consequently, any oil, or grease material in the plant can be affected by the detergent, namely the plant cells. Plant cells have two boundaries around the cell; a cell membrane on the inside and a cell wall on the outside of the cell membrane (see figure 3).
Consequently, any oil, or grease material in the plant can be affected by the detergent, namely the plant cells. Plant cells have two boundaries around the cell; a cell membrane on the inside and a cell wall on the outside of the cell membrane (see figure 3).
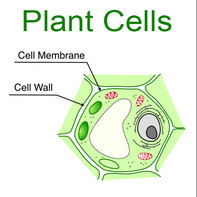 Figure 3: Plant cells (Boman, 2012)
Figure 3: Plant cells (Boman, 2012)
The cell wall functions to provide a 'skeleton' structure to help support the plant cell's shape (Rickard, 2011, p.49) and is porous to allow nutrients to flow in and out of the cell (Saupe, 2011). The cell wall is made of many different types of molecules and some of these are summarised below:
- Cellulose
- Cross-linking glycans
- Gel-like polysaccharides
- Lipids (see figure 4) (Saupe, 2011)
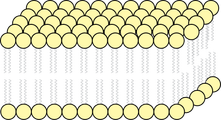 Figure 4: Lipid Bilayer (Walker, 2006)
Figure 4: Lipid Bilayer (Walker, 2006)
Lipids are usually two layers of molecules which have a similar structure to detergent molecules; a hydrophilic head (seen as yellow circles in figure 4), and hydrophobic tails (grey lines in figure 4). These molecules share similar characteristics to oils and grease due to their long hydrophobic tails and so are expected to be disrupted, and perhaps broken down by the introduction of detergents to the surrounding water supply of the plant.
To quantify the health of the plant samples during the investigation, the plant samples will be weighed, as it is expected that as the cell membranes and cell walls become damaged, these plant cells would empty their contents into the surrounding water, making the plant lighter. In addition, the plants will be closely observed and photographed for any changes in colour to indicate changes to their health during the 7 day duration. It is expected that the plants submerged in the highest concentration of detergent solution will show the fastest decline in health and weight.
Hypothesis
|
If the amount of detergent added to the plants' water supply is increased, then the mass of the plant will decrease and the colour of the plant will become less healthy, because the detergent will break down the lipids in the plant cells and destroy some of the plant cells.
|
The design of this investigation is a cause and effect experiment, so the hypothesis will follow:
If____ (is in/decreased), then _____ (will in/decrease), because ______. |
Materials
|
|
Method
|
Methods must be written in:
They should also be as detailed as possible such that the experiment can be replicated exactly by someone else. |
Results - work in progress
|
Table 1: Initial plant mass
|
Table 2: Changes to plant health
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion - work in progress
|
Points to cover in the discussion:
|
Conclusion
|
The aim of the investigation was to determine the effect of detergents in the water supply of plants on the health of the plant. It was hypothesised that when the concentration of detergent was increased in the water supply that the health of the plant would deteriorate resulting in decrease in mass, as well as changes in colour. The findings indicated ________, which _______ the hypothesis.
|
Points to cover in the conclusion:
|
References
- Northeastern University 2009, 'Computational Modeling of the Intenstinal Drug Delivery Environment', Advanced Drug Delivery Research (ADDRES) Laboratory - Northeastern University, Boston, viewed 12 February 2014, <http://www1.coe.neu.edu/~rebecca/modelling_drug_delivery_enviroment.html>.
- Rickard, G., et al. 2011, Pearson Science 8 Student Book, Pearson Australia, Melbourne.
- Roland.chem 2006, Tenside haben hydrophile und hydrophobe Enden, viewed 12 February 2014, <http://en.wikipedia.org/wiki/File:TensideHyrophilHydrophob.png>.
- Saupe, S 2011, 'Plant Physiology - Cell Walls', Plant Physiology (Biology 327), College of St. Benedict & St. John's University, Collegeville, viewed 12 February 2014, <http://employees.csbsju.edu/ssaupe/biol327/lecture/cell-wall.htm>.
- Taylor, N. et al. 2007, Study On: Chemistry 1, John Wiley & Sons Australia, Milton.
- Zumdahl, S. S. & Zumdahl, S. A. 2000, Chemistry, 5th edn, Houghton Mifflin Company, Boston.

