Naming & Writing Ionic Formulas
|
Everything that you need to know about writing and naming ionic formulas; from the simple binary ionic formulas, to the more complex formulas involving metals of variable charges from the transition metals block.
Scroll down the page to see all three videos and the extended explanations and practice problems. |
Binary Ionic Formulas
|
How to name basic binary ionic formulas; formulas that involve only two elements; a cation and an anion. In addition, I introduce the cross-over rule which is handy for situations when it is difficult to determine the right ratio of cations to anions in a formula.
|
Simon Boman 23 Feb 2014
|
Polyatomic Ionic Formulas
|
Moving on from the basics of binary ionic formulas; it is common to encounter ionic compounds whose ions are composed of more than one atom; polyatomic ions. In this video I explain how these are written differently in formula when multiple polyatomic ions are needed, and also how to recognise them.
For more information on polyatomic ions; click here to see my other page about them and an interactive activity that you can play with to help memorise them. |
Simon Boman 23 Feb 2014
|
Ionic formulas with variable charge
|
How to write the names of ionic formulas that contain elements of variable charge (the transition metals, and other metals). Also, how to write the formulas from these names.
|
Simon Boman 20 Feb 2014
|
What are the transition metals and other metals?
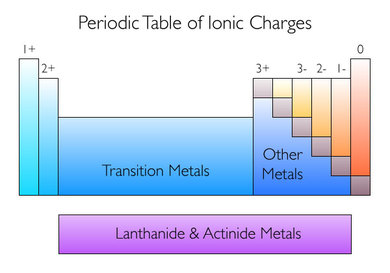 Figure 1: Periodic Table of Ionic Charges (Boman, 2014)
Figure 1: Periodic Table of Ionic Charges (Boman, 2014)
The transition metals reside in the middle of the periodic table; kind of in the 'sunken' park of it, and the other metals are to the right of this, residing just underneath the staircase divider between the metals and non-metals (see Figure 1).
These metals have a chemical property that their electron configurations are more complex than the rest of the elements and so can have a number of possible oxidation states (1+, 2+, 3+, etc, charge). This introduces some complexity to writing the names of ionic compounds that contain them. For example, when you write; Iron Oxide, do you mean Fe²⁺, or Fe³⁺?
Using Roman Numerals
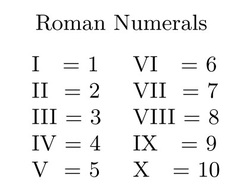 Figure 2: Roman Numerals Table (Boman, 2014)
Figure 2: Roman Numerals Table (Boman, 2014)
To specify the oxidation state of these metals we use Roman Numerals (see figure 2) within brackets next to the metal's name. For example,
- Fe²⁺ would be Iron(II)
- Fe³⁺ would be Iron(III)
- Cu¹⁺ would be Copper(I)
- Cu²⁺ would be Copper(II)
- Sn⁴⁺ would be Tin(IV)
Deducing the oxidation state from ionic formula
To apply this to ionic formulae we need to work our way backwards to determine the oxidation state of the metal. Similar to a detective; examine the clues and deduce the answer. Here is the process:
- Examine the formula, and identify the transition/other metal, and identify the non-metal
- Determine the ionic charge of the non-metal, as these will be easily identified from their location on the periodic table (see Figure 1).
- Calculate the total negative charge of the anions; (e.g. if there are 3 sulphide ions (S²⁻) then 3x2- = 6-)
- Knowing that the ionic formula is neutrally charged overall, deduce the total positive charges (e.g. if total anion charge is 6- then total cation charge is 6+)
- Divide this total number of positive charges by the number of cations present (e.g. if there are 2 iron cations (Fe) then 6+ / 2 = 3+ each)
- Write the ionic formula with that oxidation state (Fe3+ = Iron(III) )
For example; CuO, the copper is a transition metal, so its oxidation state can be anything, however oxygen in this ionic compound is the ion oxide, which has a 2- charge due to its placement on the periodic table (see figure 1, also click here to see my other video on ions for more info). Since there is only one oxide ion present, we won't need to multiply as per step 3, but we can deduce that since there are 2- charges, there must also be 2+ charges which can only be from the single copper ion present. Therefore, Copper is 2+ charge, and is written as Copper(II) Oxide.
|
Practice: Junior Science level
Write the ionic formulas for the following combinations of ions
Write the ionic formulas for the following combinations of elements (note, you will need to determine the ionic charge that each would form first)
Write the ionic formulas for the following combinations of polyatomic ions:
|
Practice: Senior Science level
You should be able to complete all of the junior science level questions, along with the following questions: Write the names of the following ions
Write the ionic formulas for the following multivalent ionic compounds:
Write the ionic formulas for the following polyatomic multivalent ionic compounds:
|

