Ionic Bonding basics - Part 2
How can you tell whether a given chemical formula is an ionic compound or not? How can we derive their names?
This video continues on from the previous video on the basics of ions, and seeks to answer these questions with some simple examples. These are some examples that you might expect in a junior science class, however a future video will help explain some of the more advanced name/formula situations, as well as their physical properties.
Simon Boman 11 Feb 2014
This video continues on from the previous video on the basics of ions, and seeks to answer these questions with some simple examples. These are some examples that you might expect in a junior science class, however a future video will help explain some of the more advanced name/formula situations, as well as their physical properties.
Simon Boman 11 Feb 2014
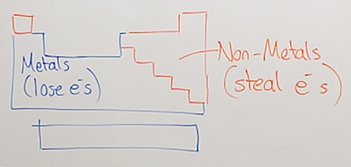
How to identify an ionic compound?
Ionic compounds are made of ions; cations and anions bound together by electrostatic attraction (opposite charges attract); this requires a combination of metals and non-metals. This is because metals tend to give their valence (outer shell) electrons away, making them positively charged ions, and non-metals tend to steal electrons to complete their valence shell (outer electron shell), making them negatively charged ions. This method will not always work as there are some exceptions like steel (Carbon & Iron), but will work for most compounds.
Practice identifying ionic compounds from the list below, using only a periodic table:
Another method of identifying ionic compounds is to examine their physical properties; however this will be covered in a later video.
Ionic compounds are made of ions; cations and anions bound together by electrostatic attraction (opposite charges attract); this requires a combination of metals and non-metals. This is because metals tend to give their valence (outer shell) electrons away, making them positively charged ions, and non-metals tend to steal electrons to complete their valence shell (outer electron shell), making them negatively charged ions. This method will not always work as there are some exceptions like steel (Carbon & Iron), but will work for most compounds.
Practice identifying ionic compounds from the list below, using only a periodic table:
- CH₃COOH
- H₂O₂
- KMnO₄
- KNO₃
- Bronze (Sn + Cu)
- HF
- CaCO₃
- MgSO₄
- CH₄
- Al₂O₃
Another method of identifying ionic compounds is to examine their physical properties; however this will be covered in a later video.
