Cleaning with vinegar and bicarb of soda
By Simon Boman, BSc/Ed. 10th September 2013
Abstract
|
When it comes to cleaning the surfaces of tiles, grout, and faucets around the home, vinegar and bicarb of soda are two of the most common cleaning agents recommended on the internet. The science of how these cleaning agents work, however, remains unclear and unsubstantiated with principles of chemistry and rigorous scientific testing to verify their effectiveness. This investigation seeks to examine the evidence against the claims; both on the effectiveness, and the explanations.
|
Aim
To investigate the explanations of how vinegar and bicarb acts upon tile grout and other surfaces to remove stains.
Hypothesis
If the amount of base (bicarb) is increased per fixed volume of vinegar (an acid), then the pH of the solution will be increased and hence neutralised, because acid and bases chemically neutralise each other.
Introduction
When acetic acid (CH₃COOH), commonly known as vinegar, is mixed with sodium carbonate (Na₂CO₃), also known as bicarb, the two chemicals combine in what is known as a neutralisation reaction between an acid and a base.
2CH₃COOH (aq) + Na₂CO₃ (s) → 2CH₃COONa (aq) + H₂CO₃ (aq)
This produces Sodium acetate (CH₃COONa), and Carbonic Acid (H₂CO₃). The carbonic acid is claimed by HowStuffWorks.com to be 'a weak acid that boosts the corrosive action of vinegar' (Halvorson & Venzon, 2006). The verification of this claim will be investigated using a pH meter attached to a data logger such that the pH changes over time can be found.
2CH₃COOH (aq) + Na₂CO₃ (s) → 2CH₃COONa (aq) + H₂CO₃ (aq)
This produces Sodium acetate (CH₃COONa), and Carbonic Acid (H₂CO₃). The carbonic acid is claimed by HowStuffWorks.com to be 'a weak acid that boosts the corrosive action of vinegar' (Halvorson & Venzon, 2006). The verification of this claim will be investigated using a pH meter attached to a data logger such that the pH changes over time can be found.
Risk Assessment
| Equipment/Chemicals | Risks involved | Management |
|---|---|---|
| Vinegar | - Corrosive to the skin and eyes | - Protective glasses |
| Bicarb of Soda | - Limited caustic properties - Possible irritant to the lungs if inhaled | - Take care not to inhale the dust |
| Glassware | - Glass breakage - Cuts from broken glass | - Handle glassware with care - Select and use crack-free glassware - Dispose of broken glass into a sharps bin |
Materials
- Homebrand vinegar
- Bicarb of soda (Sodium Bicarbonate)
- pH meter
- LabQuest data logger
- 2 x 50mL beakers
- Distilled water
- Paddle pop stick
Method
|
The temperature probe was connected to the LabQuest data logger, and the settings were changed to measure the pH over a duration of 3 minutes with a sampling rate of 60 per minute. The temperature probe was placed in a 50mL beaker of distilled water to rinse the probe before use. If the temperature probe did not produce a pH reading in the acceptable range of pH 6-8 for distilled water, then the temperature probe would be calibrated as per the instruction manual. Once the temperature probe had been rinsed, it was then placed into another 50mL beaker containing 40mL of homebrand vinegar solution and the data logger was activated for recording pH. The probe was left to sit momentarily to allow the pH level reading of the vinegar to stabilise before the addition of bicarb of soda which was administered to the beaker of vinegar via the paddle pop stick of approximately 1/8th of a teaspoon as to not encourage the resulting reaction to overspill the beaker. The bicarb of soda was then stirred into solution to ensure a thorough mixture before the solution was allowed to stand still for the remainder of the 3 minutes of recording time.
|
Figure 1: Diagram of apparatus assembly (Boman, 2013)
|
| Independent variable | Dependent variable | Controlled Variables |
|---|---|---|
| - Addition of bicarb of soda | - pH of the solution | - Temperature (standard lab conditions) - Pressure (standard lab conditions) |
Results
|
Analysis
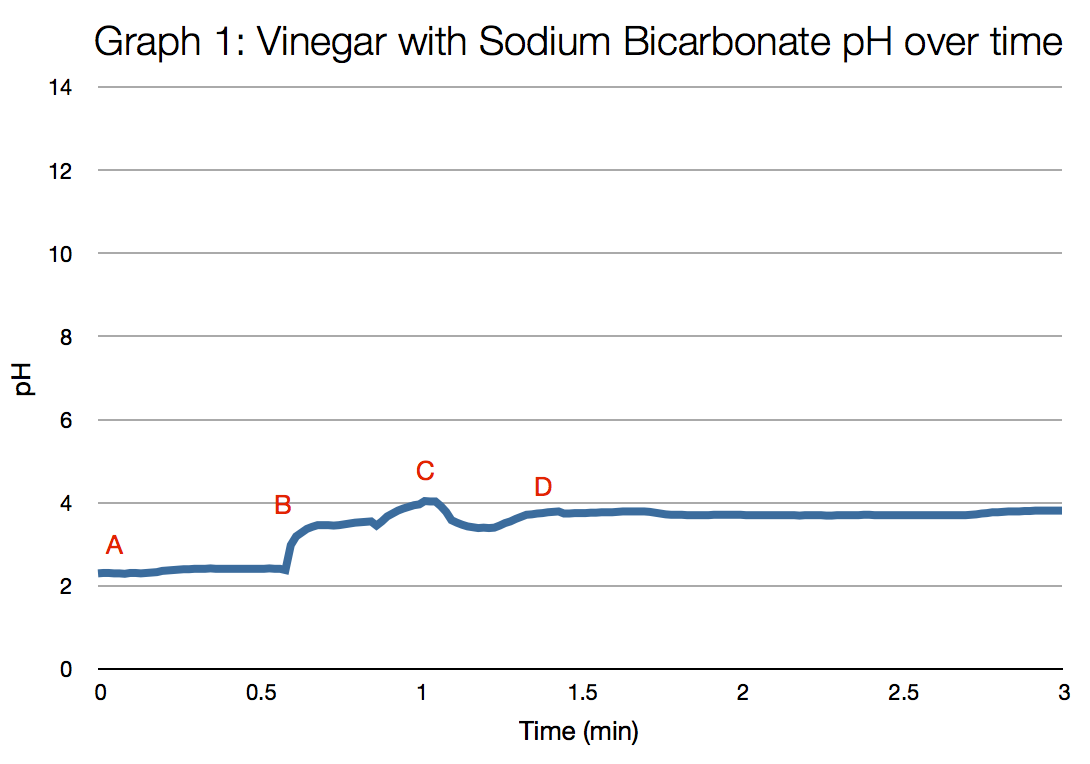
A) The pH meter was set in the vinegar and allowed to stabilise to provide a baseline average pH of the vinegar B) The bicarb of soda was added to the vinegar and stirred briefly using the wooden paddle pop stick. C) The paddle pop stick was removed and the solution was then stirred with the pH probe itself. D) The pH probe and solution was left to stand to allow the pH to stabilise. |
Discussion
Conclusion
References
Halvorson, C., & Venzon, C. 2006. HowStuffWorks "10 Uses for Baking Soda: Guidelines for Cleaning Your Bathroom". [ONLINE] Available at: http://home.howstuffworks.com/household-hints-tips/uses-for-baking-soda-cleaning-your-bathroom-ga.htm#page=1. [Accessed 10 September 13].